Miele XLIF
Monitoring
Lower extremity EMGs alone. Miele does not routinely use SSEPs.
Equipment
You will need loupes and lead for this case.
Preparation
- Know where the metal cross is, verify that it is available. This is used for localization later in the case but now is the time to make sure you know where it is.

- Verify that the C arm is in the room, plugged in, and ready to go
- Verify neurophys is in the room and is set up for lower extremity EMGs
- Anesthesiology with gas only, no paralysis
- Place Foley
- Slide table to the head all the way, arm board as high as possible, bracket for retractor holder as low as possible. This is to accommodate the c arm. You don't want to bump into the bed post.


Positioning
Positioning is lateral. Which side is up will depend on the patient's pathology and the attending's preference.
- Call for C arm as you begin positioning
- Pull the patient onto their side.
- Do not pull the patient to the edge of the bed like you would for an LP or lumbar drain. Leave at least 3 inches of space between the patient's back and the edge of the bed. There is a radiopaque portion of the bed at the lateral edge that would interfere with lateral shots if the patient were pulled all the way to the edge.

- Move the patient north or south in the bed until the top of the iliac crest is at the break in the bed.

- Place an axillary roll
- Bend the knees up slightly and pad the underside of the dependent knee and in between the knees and ankles
- Place pillows between the arms then tape loosely to the arm board
- You may be used to using blue towels to protect the skin from the tape. Miele uses clear Ioban for this purpose instead.
- Place on the iliac crest with the top border just shy of the top of the crest
- Place on the rib cage with the bottom border of the Ioban well shy of the bottom of the ribcage.
- Use 3-inch tape to secure the iliac crest and ribcage to the bed.
- Visually assess if the patient is leaning towards their belly or back.
- Start the tape on the side that the body is leaning towards in an attempt to correct them to neutral.
- Go around 3 times.
- Make sure this is tight. In fact, the tape over the ribcage will compress the cage somewhat and this is okay as long as anesthesia isn't having difficulty ventilating the patient.
- Break the bed in this order:
- Reverse Trendelenburg (may have to press the Trendelenburg button if the bed is reversed)
- Head down (may have to press legs down if the bed is reversed)
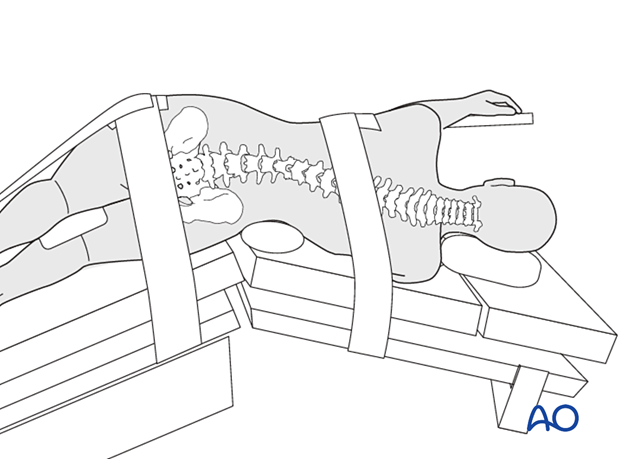
- Use 3-inch tape to pull the iliac crest to the bottom of the bed. Make sure to pad the head of the fibula using a small square of purple egg-crate foam torn from the small foam. Don't use the whole purple foam because it will prevent the tape from sticking appropriately to the leg. Start tape on the crest, go to the foot of the bed, then bring the tape back up to the crest.
- This is important: after positioning is complete, secure the Foley to the underside of the bed and do not let it dangle. This is to keep it out of the way of the C-arm which can catch a dangling Foley and traumatize the urethra. A traumatized urethra can result in hematuria. Blood in the Foley at the end of the case will make you wonder whether you injured the ureter during surgery.
Localization
Put on your lead and grab a Sage wipe, marking pen, the metal cross, and the bed control.
- Wipe the flank with a Sage wipe
- Start with an AP shot. With the bed control, tilt right or left to center the spinous process of the level of interest between the pedicles. Do not move the C arm from the neutral position (i.e. parallel to the floor). No need to align end plates at this stage. If an excessive amount of bed tilt is required to achieve a neutral plane at this step, you may have to take down the tape, realign the patient, retape, and start over.




- Next, go to a lateral shot. The goal here is to plan the incision.
- Move the bed in a Trendelenburg or reverse Trendelenburg direction to align the end plates for a true lateral. Do not move the C arm from the neutral position (i.e. perpendicular to the plane of the floor).
- Use the metal cross to localize
- For a single level, localize to the dead center of the disc space

- For multiple levels, you will still aim for the center in the anterior-posterior direction, but now for the middle of the planned levels in the rostral-caudal direction.
- Mark an approximately 3 cm incision parallel with the disc space
- For a single level, localize to the dead center of the disc space
Prep
Two chlorhexidine preps.
Draping
Four blue towels in a square, down sheet, Ioban, and a spine drape.
All cords go off the foot of the bed except for the light cord which goes off the top. Be sure to leave the cord for the dilator stimulator shorter than the others.
Attach the retractor arm to the bed.
Attach the C-armor.

Ask for two stacked stools on each side of the patient for the main surgeon and the assistant.

Approach
Make skin incision with a #10 scalpel blade. Go to the level of the subcutaneous fat and no deeper.
Use the Bovie to cauterize any bleeding. Once the skin incision is dry, Miele removes the tip from the Bovie and hands it back to the scrub tech. It will not be used again. Use of the Bovie in the muscle can denervate the muscle and result in unsightly bulging and a cosmetic defect.

Use your longest and strongest finger (mine is my middle) to poke through each of the three layers of the abdominal wall (external oblique, internal oblique, and transversus abdominis) to arrive at the retroperitoneal space. Apply downward pressure with wide sweeping finger rotation to push through and split the fibers of the abdominal musculature.


Carry dissection in the retroperitoneal space until you feel the transverse process of a vertebral body. As the lateral-most projection of the vertebral body, the transverse process is the structure you are most likely to encounter first.
Sweep your finger anteriorly to the lateral aspect of the vertebral bodies and feel for the bony ridge of the disc space.
Keep your finger in the patient with your palm facing you and pass the smallest dilator along the palmar surface of your finger until it hits the bone. Your finger pushes the peritoneal contents anteriorly and protects them from the dilator.
With the smallest dilator docked on bone, remove your finger, hold the dilator with an instrument, and take a lateral shot.
Use small micro movements to move the tip of the dilator to the center of the targeted disc space. The "fifty-yard line". Push a K wire through the central cannula of the smallest dilator into the disc space.
Now, don't move the tip but change the angle of the dilator to get a shot "straight down the pipe" of the dilator, i.e. where the x-ray projection of the dilator is a circle. Save this image and ask the x-ray tech to keep it up on one of the C arm screens for the rest of the case. This acts as a reference for you for the ultimate anterior-posterior location of your retractor.


Next, attach the retractor stimulator to the back end of the dilator and ask the neurophysiology tech to start stimulating at 20 mA. If they get a response, ask for a threshold. For Dr. Miele, thresholds greater than 5 mA are acceptable. Stimulate in each of the four cardinal directions (posterior, superior, anterior, and inferior). If you consistently get thresholds <5 mA you may need to remove the K wire and reposition the dilator.
While you are stimulating, look at the side of the smallest dilator to see how deep of a retractor to ask for. The scrub tech can be loading the appropriate length retractor blades while you finish stimulating.

If thresholds are acceptable, turn the C-arm to an AP then proceed with passing the remaining dilators over the smallest, followed by the retractor itself. Hold the retractor by the blades to prevent them from coming apart while it is being placed. The handles of the retractor go posteriorly, towards the primary operator. Attach the retractor to the articulating arm, then take an AP shot.
The K wire should be at least 50% of the way into the disc space. Pay attention, also that the spinous process is still centered between the pedicles and that the patient didn't slump one way or another during the approach.

Open the retractor slightly to make space for the dilators to come out. Miele calls this "two clicks and two clicks", i.e. two clicks squeezing the handle of the retractor and two clicks pulling the third blade back with the T handle.
Remove all three dilators but keep the K wire in place. This stays in until you have the shim in. Place the lights in the retractor and turn them on.

Now look through the retractor to the bottom of the field. There will probably be some pooled blood at the bottom that you will need to suction. Look for the location of the K wire relative to the posterior blade of the retractor. If the K wire was placed in the dead center of the disc space, you will want the posterior blade of the retractor to be about 1 cm behind the K wire.
You may see the broad white ridge of the disc right away, which is ideal. You're more likely, though, to see some fibers of psoas muscle covering the disc. These can be dissected to the side with the tip of the suction or a bayoneted Penfield 4.
This is the stage where you should look for the ureter, which can be seen as a tubular structure with subtle peristaltic motion. You should also look for large nerves. If either the ureter or femoral nerve are seen, they will need to be moved and tucked behind the retractor. In the worst case scenario, you may need to move the retractor to avoid their course. Sometimes, the femoral nerve can be hidden under stray fibers of the psoas.

Ask for the direct stimulator and stimulate at 20 mA the landing zone for the shim (red arrow) as well as the planned course of the annulotomy. Thresholds above 5 mA are acceptable, especially if you can clearly visualize the disc without any suspicious structures.

If the landing zone for the shim is clear, place the shim and tap into the disc with a mallet under AP fluoroscopic guidance. The function of the shim is to hold the tip of the retractor stable against the spine and prevent rostral-caudal slippage.



Once the shim is in place, remove the K wire.
Ask for the #15 blade on a bayoneted long handle and incise the annulus. The annular incision should be long enough to accommodate the width of the Cobb elevator.
Place the Cobb in the annulotomy and tap across the disc space with the mallet. Progress will slow as you approach the contralateral annulus. With a brisk tap, pop through the contralateral annulus to the other side.

Remove the Cobb.
Prepare the end plates with sequentially larger disc space shavers, starting at 8 mm and progressing in size until you hear "gristle" when you turn it and pieces of cartilaginous end plate start to come out. Miele does not use any other form of end plate preparation like curettes.
Remove free fragments with a pituitary. Hold the pituitary "back hand" with the handle facing you. This is to avoid catching bowel that could be exposed anteriorly between the blades of the retractor.
Next, Miele uses a small rectangular trial that comes on a detachable T handle to check the proposed anterior-posterior position of the graft. Tap it into place under AP fluoroscopy then remove the T handle and switch for a quick lateral shot. Removal of the T handle helps to visualize the location of the trial and minimize surrounding background.



Go back to an AP. Remove the rectangular trial and tap in a real trial. Be sure to keep the lordotic part anterior - "fat to the front". Start with an 8 mm height trial. If that can be removed without the slap hammer, place a 10 mm height graft. Rarely, if the disc space is large or a large amount of indirect decompression is desired, Miele may choose a 12 mm height.
On the AP shot, there are subtle gradations on the side of the Depuy trial that signify the length of the trial. Use these to choose the length of your graft.

Remove the trial with the slap hammer. Follow with the pituitary to remove any new disc fragments.
Miele always uses a lateral plate. You will need to specify if you want the holes in the plate pointing towards the back or the front. If you think your graft is going to land relatively posterior, have the plate attached with the screw holes in the front. Otherwise, choose screw holes in the back.
Tap the graft into place under AP fluoroscopic guidance. Once it is in place, disengage from the applier and switch to a quick lateral to verify that the anterior-posterior location is acceptable.

Next, secure the lateral plate with screws under AP fluoroscopy. Tap the trajectory with a drill instead of an awl. This reduces the risk of vertebral body fracture.
The longest Depuy screw is 25 mm and this is the one Dr. Miele uses. He prefers a longer screw for a bicortical trajectory but those are not available with this vendor. Place the tip of the screw in the screw hole and tap the back of the applier with a mallet to engage the screw tip before screwing it the rest of the way into place. Turn the locking screw before moving on to the second screw.
If the case is multiple levels, repeat all of the above steps for each level. Collapse the retractor and remove it entirely then start over with the smallest dilator and a lateral shot.
After placement of the last cage, irrigate down the retractor with a liter of antibiotic irrigation.

Place Surgifoam at the bottom of the field and pack with a strung out Raytec on a pituitary.
Collapse the retractor and remove it slowly while looking down the barrel for any brisk bleeding.
Closure
Unbreak the bed to bring the tissue together for closure.
0 Polysorb suture for the muscle layer.
2-0 Polysorb for the dermal layer.
4-0 Quill subcuticular for the skin followed by skin glue.
No dressing.
Postop
All patients go to the floor. No blood pressure parameters. CT lumbar spine without contrast.
Notes
- If your trajectory is relatively posterior, err on the side of placing a shorter graft. If you place a long graft you run the risk of hitting the contralateral exiting nerve root.
